-
1. What are tannins?
Tannins are astringent and potentially bitter compounds found in most plant species. While there is a diversity of individual compounds, all tannins have a few things in common. Tannins are polyphenolic compounds that bind protein, which is the basis of their role in tanning hides for leather and gave rise to their name. They are also able to interact with and precipitate salivary proteins, which is the basis for astringency perception. While it is slightly more complicated than this, it serves as a good basic definition of tannins for this article.

Figure 1. Examples of the origins tannins in grapes and wine. Photo taken from Wine Folly
-
2. What role do they play in plants?
It is thought that through their protein binding capacity, tannins play two defensive roles in grapes, one against microorganisms and the other against herbivores. Tannins also bind to cell walls and other phenolics to create a defensive barrier when cell integrity is lost, as in the case of pathogen invasion. Being bitter and astringent, their taste acts as a deterrent to herbivore attack, and can also bind to ingested protein, making it unavailable as a nitrogen source, and limiting repeated consumption of the plant in question.
-
3. Where are tannins found in the grapes?
Tannins are found in the skin, seed, flesh and stem parts of the grape. Tannins that are derived from the grape skin tissues are often described as being a ‘riper’ type of tannin, which may give rise to common descriptors such as ‘dusty’ or ‘velvety’. Skin tannins are known to have a higher mean polymer length than those tannins derived from seeds.Grape seed tissues tend to give rise to tannins which are considered to be coarser, harder and grippier when describing their astringency. Bitterness for the most part appears to be primarily localised and attributed to the grape seed tissues. Grape stem tannins appear similar compositionally to both skin and seed tannins, but they are also responsible for imparting additional ‘green’ flavour components to wines which may not be considered beneficial. While tannins present in skin, seed and stem tissues are relatively extractable those present in the flesh tissues of grapes are not extracted due to their strong association with proteins and polysaccharides of the cell walls. Aside from viticultural factors which affect the relative extractability of skin and seed tannins, tannins which end up in the final wine are also highly dependent on the particular winemaking techniques employed.
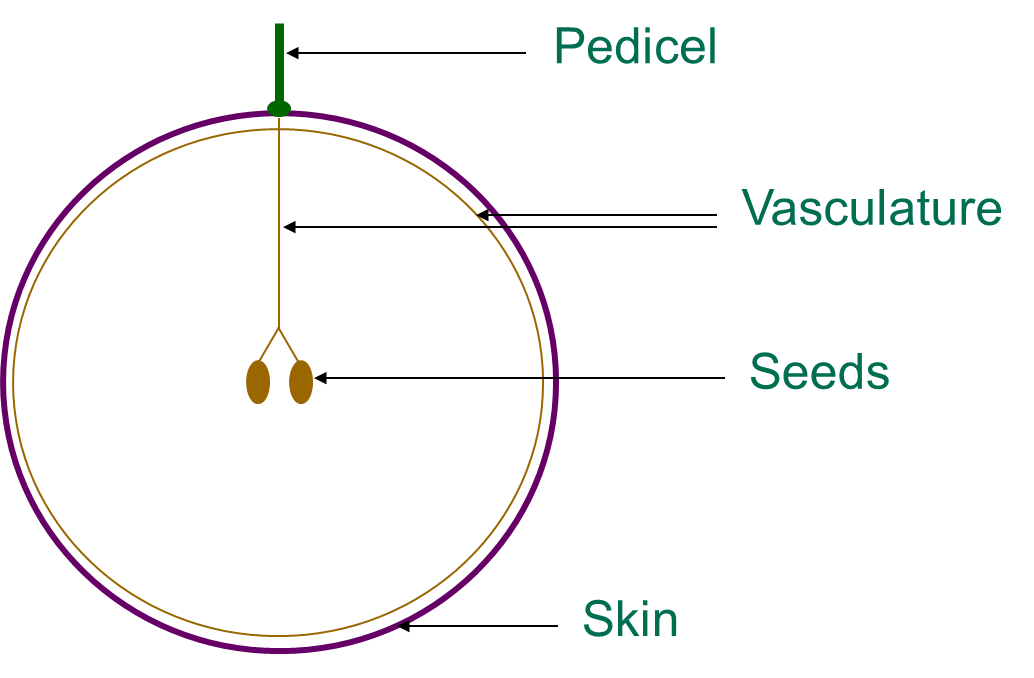
Figure 2. Where tannins are found in grapes – seeds, skin and grape stalks or pedicel are the major sources. Diagram taken from Dr Mark Downey (Agriculture Victoria)
-
4. How are tannins made in grapevines?
Tannin synthesis in grapes occurs via the same biosynthetic pathway that makes anthocyanins (red pigments in red/black grapes), flavonols (e.g. quercetin, kaempferol – natural sunscreen products) and phenolic acids (e.g. caffeic acid, resveratrol). During berry development the main phase of tannin biosynthesis occurs between flowering and veraisonSome viticultural management practices known to influence tannin levels during this flowering to veraison period are presented in a later section.
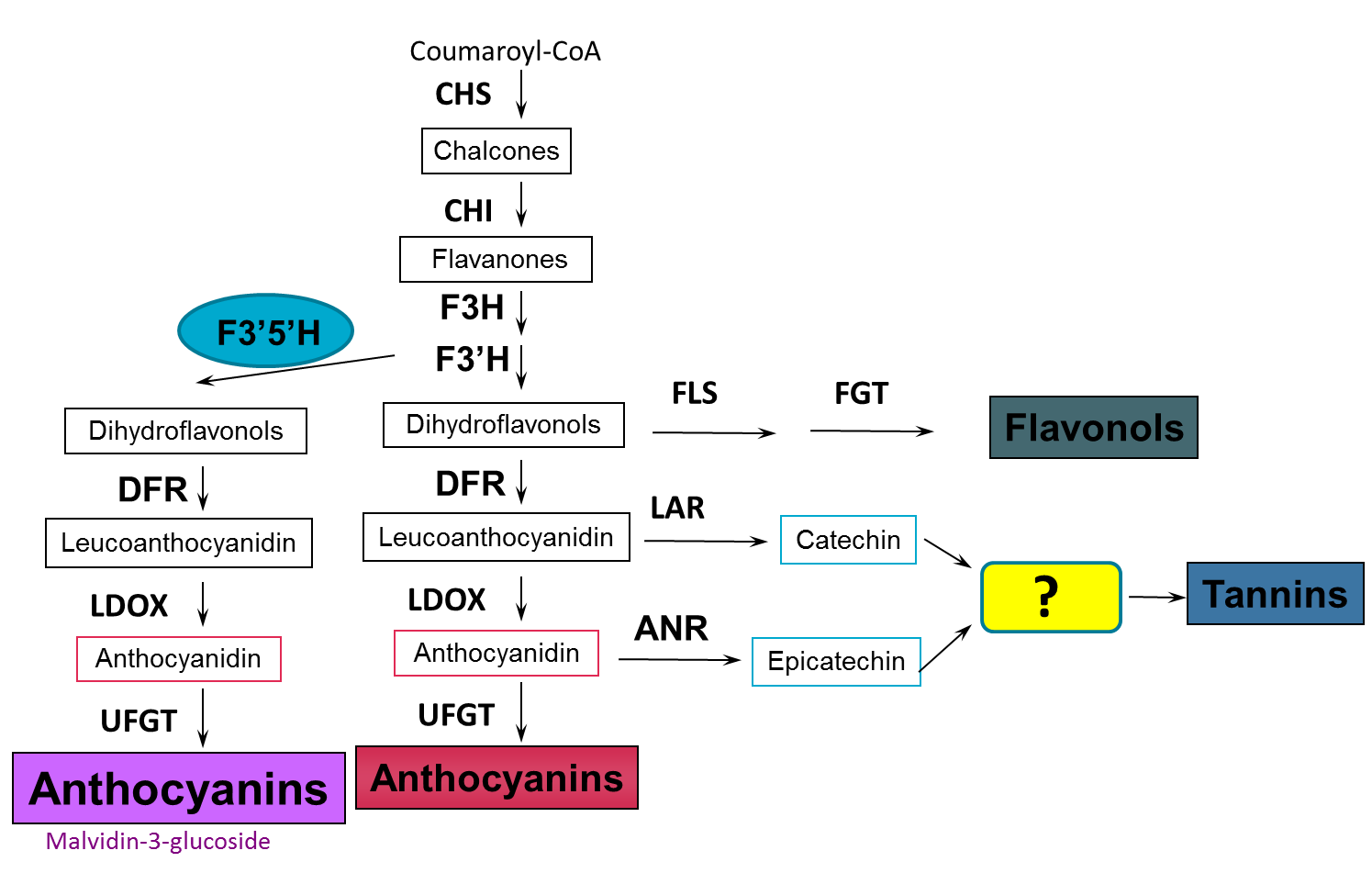
Figure 3. The tannin biosynthetic pathway in grapes. Image taken from Dr Simon Robinson (CSIRO) - Flavonoids in grapes
-
5. How do tannins change throughout ripening?
Tannins appear to decline considerably on a per-berry basis post-veraison during the second period of berry growth and development. The reduction in seed tannin content appears to be the result of oxidation as the tannins become fixed to the seed coat. As a result of this, the composition of extracted seed tannins changes considerably, and is characterised by a proportional reduction in the most bitter tannin components. Skin tannins on the other hand have been observed to show a very variable trend, and may decline, remain constant or in fact increase in the post-veraison period. During ripening skin tannins can become modified in structure, increasing in their overall size or mean polymer length during this phase, and may be modified through interactions with pectins and anthocyanins. Together, these modifications can influence the final wine quality. This makes the berry development phase of growth an important consideration in relation to overall wine texture and colour stability.
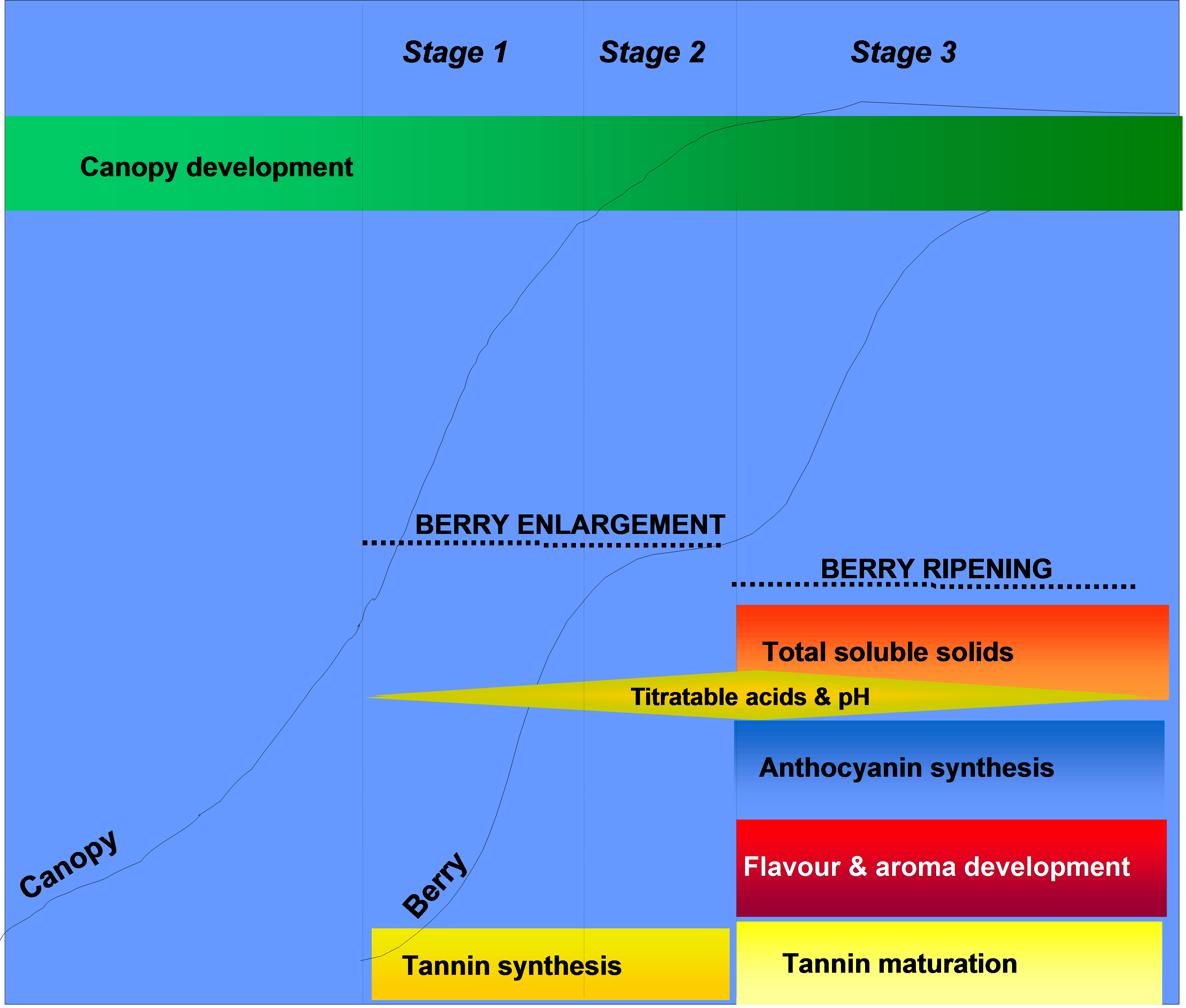
Figure 4. Accumulation of tannin during grape berry development. Note that the major period of tannin synthesis occurs between flowering and veraison, whereas tannin maturation occurs post-veraison.
-
6. Do tannins differ between grape varieties?
Anecdotal evidence suggests that tannin levels do vary substantially between different grape varieties, with some varieties considered high in tannins, e.g. Tannat and Nebbiolo, and others low in tannin, e.g. Merlot and Barbera. It is important to highlight that winemaking style greatly affects how much tannin is extracted into a wine.
Table 1. Grape varieties or wines often considered either high or low in tannin content.
High tannin varieties: Low tannin varieties: Nebbiolo Barbera Cabernet Sauvignon Zinfandel Tempranillo Pinot Noir Montepulciano Primitivo Petit Verdot Grenache Shiraz/Syrah Merlot Tannat Interestingly, a study of grape skin tannins from 36 different grape cultivars showed that many of the cultivars with high tannin levels were not those traditionally considered high in tannin, but were actually table grape varieties (e.g. Red Emperor).
-
7. Do climatic differences influence the level of tannin in grapes?
As in most other biological processes, temperature plays a significant role in the synthesis of tannins in grapes. Early in berry development (between fruit set and veraison) higher temperatures have been associated with an increase in tannin levels in berries, indicating that these warmer temperatures stimulate tannin biosynthesis. However, warmer temperatures between veraison and harvest have been shown to have little effect on tannin levels, but are detrimental to anthocyanin accumulation, through decreased biosynthesis and increased degradation of the anthocyanins compounds. While this may seem all very theoretical, it really comes back to absolute berry surface/skin temperatures, where maintenance of berry temperatures between 15oC and 35oC appears to be optimal for the enzymes involved in the flavonoid pathway responsible for anthocyanin, flavonol and tannin synthesis. The problem is in black wine-grape varieties, such as Shiraz, berries directly exposed to sunlight can be up to 15oC above the ambient air temperature. This can lead to berry temperatures on a 35oC day being at, or near 50oC, which can result in lower anthocyanin levels in grapes if exposed to these temperatures post-veraison. At these extremes in temperature, the effect on tannin synthesis is not yet known.
Large seasonal differences are often observed in grape and wine quality and composition from within the same vineyard. In many cases, the variation observed between seasons can often be larger than that observed effects between viticultural treatments within a single season. These seasonal differences can be attributed to the differences in weather patterns observed during a given growing season. Many of these seasonal differences are often attributed to differences in temperature and plant water availability.
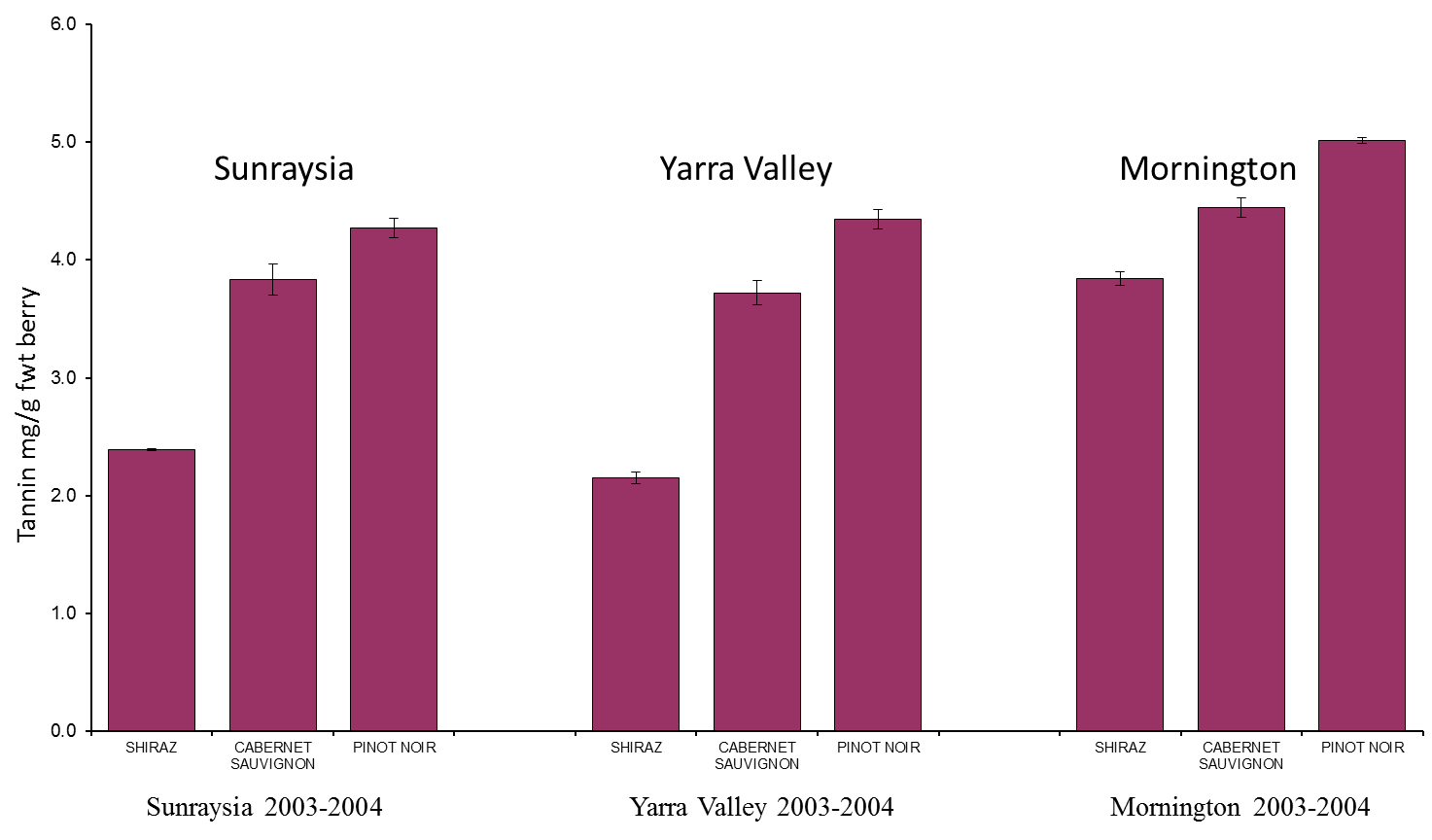
Figure 5. Grapevine variety and site differences in total tannin content. Image taken from Dr Simon Robinson (CSIRO) - Flavonoids in grapes.
-
8. What can be done in the vineyard to influence tannin levels?
Modifying bunch exposure
Canopy management and bunch exposure management techniques have long been touted as tools for managing anthocyanin/colour levels in wine-grapes. However, the results from increasing bunch exposure to direct sunlight or shading do appear to be variable and depend on the specific variety, season and site. In some varieties, increasing exposure to direct light has proven effective in increasing anthocyanin, flavonol and tannin (polymeric flavan-3-ols) levels in grapes. However, at other sites, no effect of bunch exposure has been observed.
For tannins, the general consensus now is that increased cluster light exposure, through leaf removal around the bunch zone, just prior to flowering, during flowering and early on during berry development, favours the accumulation of berry anthocyanins, flavonols and skin tannins. Tannins appear responsive to UVB radiation. This appears to be the case as long as extremes in berry temperatures (i.e. <15oC and >35oC) are not experienced. Outside these temperatures key enzymes responsible for the synthesis of these flavonoid compounds will not function effectively. Also, one would need to be mindful of the unintended consequences of significant leaf removal such an early stage of development, such as a potential reduction in the percentage fruit set and smaller berries.

Figure 6. Cabernet Sauvignon grown on VSP trellis where leaves have been plucked in bunch zone to increase bunch exposure.
Modifying irrigation levels
A number of studies have looked at the effect of irrigation on tannin levels in grapes and wine. Most of these studies have concluded that irrigation or deficit irrigation between berry set and veraison has no effect on grape tannin biosynthesis per se other than reducing berry size and thereby concentrating the amount of tannin assessed on a mg/g berry fresh weight basis, similar to the effect observed for anthocyanins. Application of a water deficit post-veraison has an even smaller effect on reducing berry size and frequently there is no observed effect on tannin concentration on a mg/g basis.
While the hydraulic effect of limiting irrigation between berry set and veraison results in smaller berries, the effect of roots growing in a drying soil may also induce an abscisic acid (ABA) mediated chemical response. The application of synthetic ABA to grape bunches pre-veraison initially depresses tannin synthesis prior to veraison (in green grapes), but stimulates anthocyanin and skin tannin accumulation post-veraison. This points to the fact that the tannin biosynthesis pathway is highly regulated in terms of berry developmental stage. To reinterpret data from irrigation trials, the ABA-mediated effect may be only short-lived in Regulated Deficit Irrigation (RDI) and Sustained Deficit Irrigation (SDI) managed vineyards, and insufficient to result in changes in biosynthesis. However, it is interesting to note that in a Partial Rootzone Drying (PRD) managed vineyard the effect of elevated ABA may be prolonged. As yet, the case for a PRD effect on tannin biosynthesis in grapes has not yet been evaluated.

Figure 7. Drip irrigation in a vineyard.
The influence of vine vigour
In a precision viticulture study of three Shiraz sites across south-eastern Australia where berry composition and many other viticultural parameters were studied, an inverse relationship between tannin concentration in the grapes and vine vigour was observed. High vigour vines tended to have lower tannin levels while low vigour vines had higher tannins. This finding was consistent with similar research conducted on Pinot Noir in Oregon, USA suggesting that vine vigour was the major driver of tannin variability across an individual vineyard.
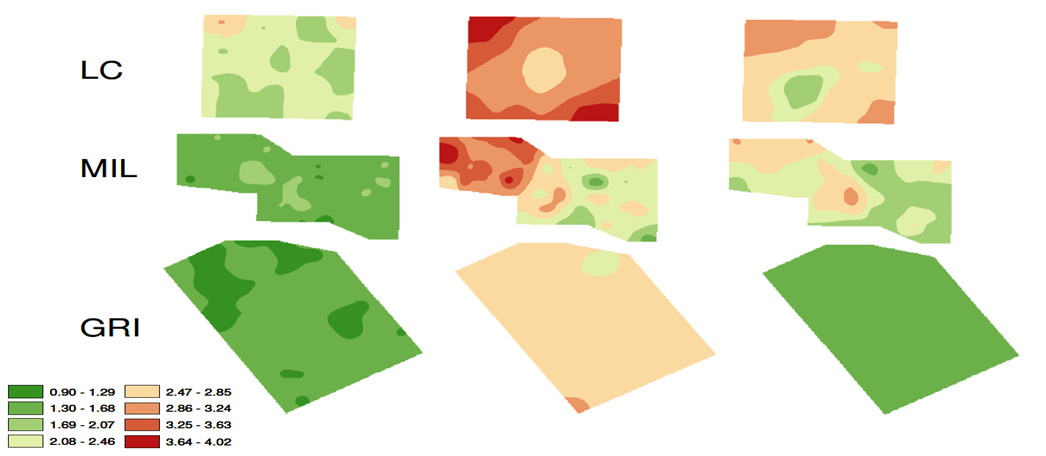
Figure 8. Tannin variability (mg/g berry) across three Shiraz vineyards located in Langhorne Creek (LC), Mildura (MIL) and Griffith (GRI) across three different growing seasons, Season 1 (2004-05), Season 2 (2005-06) and Season 3 (2006-07).
Influence of plant growth regulators or elicitors
As mentioned earlier, ABA can stimulate the production of skin tannin in grape berries if applied in an exogenous form around veraison. In contrast to this, if exogenous auxins are applied, such as naphthalene acetic acid (NAA) just prior to veraison, it is possible to actually delay ripening and the onset of colour change and depress the production of skin tannins in grape berries.
Other plant elicitors such as methyl jasmonate (MeJ) or benzothiadiazole (BTH) can mimic plant defense response to pathogen attack, triggering the production of salicylic acid in the vine, which in turn stimulates the flavonoid biosynthetic pathway, leading to enhanced production of anthocyanins, flavonols and skin tannin, as well as increasing the mean polymer length of the skin tannins. It does not appear to stimulate the production of tannin in seeds, which is ideal. The real benefit of this approach is that it can be applied later in grape development, after veraison, and still produces a response. While these plant elicitors MeJ and BTH may be available, they are not currently registered for use for wine-grape production in Australia. This is an area that requires further research and development before becoming commercially available in Australia.
The influence of grape ripeness on tannin levels
As mentioned previously, as grapes ripen, changes in total levels of skin tannin can be variable. However, there appears to be a general increase in extractable skin tannin concentration as opposed to total skin tannin, which usually, but not always, results in higher concentration of wine tannin, mainly due to extraction from the skins. Higher levels of anthocyanin have been shown to facilitate tannin extraction, so this may in part be due to the general increase in the level of anthocyanins during ripening, which may facilitate tannin solubility and increase extraction of skin tannin from grapes into the wine. A further possibility explaining this phenomenon is that higher sugars are associated with ripening, which, as they are converted to higher alcohol levels may indirectly be associated with improvements in extraction.
In a recent study conducted on Shiraz growing in both the Barossa and Murray Valley (Sunraysia) regions, tannin extraction was found to vary substantially by site and season throughout maturation. While there was a trend for tannin extractability to increase with increasing maturity in Shiraz grapes grown in the Barossa, tannin extraction decreased at the later harvest date in grapes grown in the Sunraysia. This again highlights the variable results that can be achieved as a result of variety, site and growing season.

Figure 9. Bunch of grapes close to harvest
-
9. Why are tannin levels in grapes and wine often not related?
While wine tannin primarily originates from the tannin present in the grapes (excluding tannin derived from oak treatment and exogenous additions of tannin), the journey from grape to wine is a complex one. Grape tannin needs to be extracted from solid grape material into must during fermentation, and then undergoes chemical rearrangements to reach its final form in wine. This is why it is not simple to predict final wine tannin by simply measuring grape tannin. These discrepancies between grape tannin and wine tannin result in part from interactions between tannins and grape cell wall material. In the seed, tannins are located in the seed coat and this layer of cells dies as the seed matures. When this occurs, tannins bind to other phenolics and cell wall proteins and polysaccharides. In grape skin, tannins have been thought to be located in vacuoles in a similar manner to anthocyanins. However, there is a body of evidence that indicates that tannins bond to the cell walls. Recent studies have isolated cell wall material and measured tannin showing that as much as 50% of tannin could be located in the cell wall. The presence of tannin in the cell walls may also account for the apparent decrease in tannins sometimes observed during berry development. The development of the ‘extractable grape tannin’ (EGT) assay at AWRI has improved understanding of the relationship between grape tannin and wine tannin. The assay measures tannin extracted in dilute alcohol, and mimics the interactions which would eventuate during crushing and winemaking, including binding to cell walls and possibly precipitation by endogenous grape proteins. Using grapes sourced from South Australia, a strong prediction of wine tannin concentration was found for Cabernet Sauvignon and Shiraz grapes using the EGT assay. Further work is needed to understand how this can be applied to different viticultural contexts and cultivars within Australia. For more information on AWRI’s wine-like extractable tannin method please refer to Measuring extractable tannin fact sheet (Note: this document does not meet WCAG 2.0 accessibility guidelines)
-
10. But you can add tannin to wine right?
While winemakers recognise there are tannins in grapes, often the level extracted from the grapes is not sufficient for a particular wine style, so it is common practice to add tannin to wine during fermentation or ageing. The main reasons for adding tannin are to stabilise colour or to alter mouth-feel. The tannins that are added to wine can come from oak barrels, chips and staves (hydrolysable tannin), or from the addition of oenotannins. However, commercially available oenological tannins generally contain around 12-48% tannin and may have little impact on wine when added at recommended addition rates. While recommended addition rates may be too low to affect the measurable amount of tannin in wine, excessive addition rates may have a negative impact on wine sensory characteristics. For more information on the addition of exogenous tannins to wine - wine research busts tannin additions myth
.
-
11. Tannins: summary
Research over the past 10-15 years has provided many valuable insights into the flavanoid biosynthetic pathway and the genes/regulators responsible for tannin, anthocyanin and flavonol synthesis in grapes – Vitis vinifera L. Understanding how tannins in the grape translate to tannins in the wine is complex, but the recent development of AWRI’s EGT assay using the 15% ethanol extraction appears to be an important step in bridging the gap between research and industry adoption of routine tannin measurements.
Vignerons can manage tannins at every stage of production, from the vineyard right through to the glass. Variety, site selection, climate, season, canopy management, bunch exposure, vine vigour management, irrigation and fruit maturity all play an important role in defining the level of tannin and tannin composition, with a focus on optimising skin tannins, at harvest. Winemakers can then use their extensive toolkit to optimise extraction of the desirable tannins to achieve the best balance, length, intensity, complexity, integration and astringency in the finished wine.
-
12. Acknowledgments
This webpage includes some of the research undertaken by the anthocyanin and tannin research team located at Agriculture Victoria/Department of Economic Development, Jobs, Transport and Resources (DEDJTR) in Mildura, Victoria over the past decade, contributing to the understanding of anthocyanins and tannins in winegrapes. The author also wishes to acknowledge Dr Keren Bindon (AWRI) for all her guidance and assistance in assembling this article.
-
13. Further Reading
Downey, M.O., Dokoozlian, N.E., Krstic, M.P. 2006. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: a review of recent research. Am. J. Enol. Vitic. 57(3): 257-268.
Downey, M. 2010. Tannin management in the vineyard. Grape and Wine Research and Development Corporation (GWRDC) Innovators’ Network Newsletter May 2010: 6 pages.
Kilmister, R. 2015. Identifying vineyard and winery management practices that impact on tannin extraction. Final report to Australian Grape and Wine Authority, project DPI1402 June 2015, 63 pages.
Scrimgeour, N., Smith, P., Bindon, K., Wilkes, E. 2015. New tool shed light on relationship between grape and wine tannins. Aust. N.Z. Grapegrower Winemaker 613: 61-63.
Scrimgeour, N.; Bindon, K.; Wilkes, E.; Smith, P. and Cynkar, W. 2014. Unravelling the relationship between grape and wine tannin and colour. Wine Vitic. J. November/December: 28-32.
Smith, P.; Bindon, K.; McRae, J.; Kassara, S. and Johnson, D. 2014. Tannin: impacts and opportunities along the value chain. Wine Vitic. J. March/April:38-41.
Fact Sheet: Measuring-extractable-tannin-fact-sheet Download PDF in new window (Note: this document does not meet WCAG 2.0 accessibility guidelines)
Industry Networks
Tannins in grapes and wine
- 1. What are tannins?
- 2. What role do they play in plants?
- 3. Where are tannins found in the grapes?
- 4. How are tannins made in grapevines?
- 5. How do tannins change throughout ripening?
- 6. Do tannins differ between grape varieties?
- 7. Do climatic differences influence the level of tannin in grapes?
- 8. What can be done in the vineyard to influence tannin levels?
- 9. Why are tannin levels in grapes and wine often not related?
- 10. But you can add tannin to wine right?
- 11. Tannins: summary
- 12. Acknowledgments
- 13. Further Reading
